Abstract
Introduction. Recent FDA approval of idecabtagene vicleucel (ide-cel), a chimeric antigen receptor (CAR) T-cell therapy for relapsed/refractory multiple myeloma (RRMM), offers renewed hope to a patient population with incurable disease. Although there were noteworthy treatment responses observed in the pivotal KarMMa clinical trial of ide-cel, racial and ethnic minority groups were underrepresented, and little is known regarding the safety and efficacy of ide-cel in diverse patient populations. To address this critical gap in knowledge, we used data from a newly formed consortium of institutions treating high volumes of RRMM patients with standard-of-care (SOC) ide-cel, the U.S. MM Cellular Therapy Consortium, to investigate racial and ethnic differences in safety, efficacy, and outcomes among patients treated with SOC ide-cel.
Methods. Data were pooled from 215 RRMM patients treated with SOC ide-cel across 11 sites. Chi-square and Kruskal-Wallis rank sum tests were used to investigate differences in patient and clinical characteristics, standard inflammatory laboratory values (e.g., C-reactive protein [CRP], ferritin), immune-mediated toxicities (cytokine release syndrome [CRS], immune effector cell-associated neurotoxicity syndrome [ICANS]), cytopenias, and treatment responses by race and ethnicity. Kaplan-Meier survival curves and log-rank tests were used to examine overall survival (OS) and progression-free survival (PFS) by race and ethnicity.
Results. Among the 215 RRMM patients treated with SOC ide-cel, the median age at infusion was 64 years (range, 36 to 83 years) and 34% of patients had high-risk cytogenetic abnormalities. Forty-five percent of patients had extramedullary disease and 43% had penta-refractory disease. The median number of prior lines of therapy before ide-cel infusion was six (range, 3 to 19). About 76% of patients would have been excluded from the KarMMa trial that led to FDA approval of ide-cel. Despite this, the best overall response rate (ORR) was 84% and 34% of patients achieved a complete response (CR) or better. Of the 83 patients with a CR or better, 66% were minimal residual disease negative.
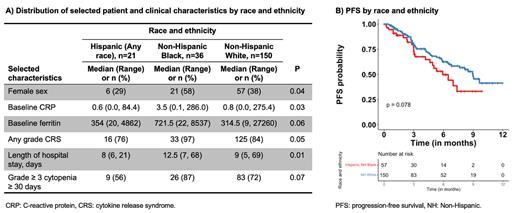
Seventy percent (n=150) of patients self-identified as Non-Hispanic White, 17% (n=36) as Non-Hispanic Black, 10% (n=21) as Hispanic, and 3% (n=8) as Asian, Pacific Islander, American Indian, or Alaskan Native. For downstream analyses, we excluded the latter heterogeneous group of underrepresented racial and ethnic groups due to limitations of sample size and power. Compared to Non-Hispanic White and Hispanic patients, Black patients were more likely to be female (38% vs. 29% vs. 58%, respectively; P=0.04) and have higher levels of baseline inflammatory markers, including C-reactive protein (CRP; median 0.8 vs. 0.6 vs. 3.5 mg/dL, respectively; P=0.03) and ferritin (median 315 vs. 354 vs. 722 ng/mL; P=0.06). In addition, compared to Non-Hispanic White and Hispanic patients, Non-Hispanic Black patients were more likely to develop any grade CRS (84% vs. 76% vs. 97%, respectively; P=0.05), have a longer hospital stay (median of 9 vs. 8 vs. 12.5 days, respectively; P=0.01), and experience severe (i.e., grade ≥ 3) prolonged cytopenias (≥ 30 days post infusion; 72% vs. 56% vs. 87%, respectively; P=0.07). No racial and ethnic differences in OS were observed. However, Hispanic and Non-Hispanic Black patients combined had worse PFS compared to Non-Hispanic White patients (median PFS of 5.9 vs. 9.0 months; P=0.08). This was likely driven by Hispanic patients, as Hispanic patients had a worse ORR compared to Non-Hispanic Black and White patients (65% vs. 88% vs. 86%, respectively; P=0.08). No other differences in patient and clinical characteristics, standard inflammatory laboratory values, immune-mediated toxicities, cytopenias, and treatment responses were observed by race and ethnicity.
Conclusion. We observed racial and ethnic differences in systemic inflammation, safety, and PFS among RRMM patients treated with ide-cel CAR T-cell therapy in the SOC setting. As a greater volume of patients are treated with CAR T therapy and follow-up time matures, we will have increased power to further investigate racial and ethnic differences in patient and clinical characteristics and clinical outcomes.
LCP and LBO contributed equally. SS, DKH, and KP contributed equally.
Disclosures
Blue:Sanofi: Consultancy, Speakers Bureau; Jassen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria. Freeman:Incyte: Honoraria; Sanofi: Honoraria; Amgen: Honoraria; Janssen: Honoraria, Research Funding; Bristol Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees. Locke:), National Cancer Institute: Research Funding; Takeda: Consultancy; CERo Therapeutics: Research Funding; Leukemia and Lymphoma Society: Research Funding; Society for Immunotherapy of Cancer: Other: Education or editorial activity; Imedex: Other: Education or editorial activity; Clinical Care Options Oncology: Other: Education or editorial activity; BioPharm Communications: Other: Education or editorial activity; CAREducation: Other: Education or editorial activity; ASH: Other: Education or editorial activity; Aptitude Health: Other: Education or editorial activity; Sana: Consultancy; BMS: Research Funding; Daiichi Sankyo: Consultancy; A2: Consultancy; Celgene: Consultancy; Other: Patents & Royalties: patents, royalties, other intellectual property from several patents held by the institution in my name (unlicensed) in the field of cellular immunotherapy.; Wugen: Consultancy; Umoja: Consultancy; Novartis: Consultancy, Research Funding; Legend Biotech: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Janssen: Consultancy; Iovance: Consultancy; GammaDelta Therapeutics: Consultancy; Emerging Therapy Solutions Gerson Lehrman Group: Consultancy; EcoR1: Consultancy; Cowen: Consultancy; Calibr: Consultancy; Cellular Biomedicine Group: Consultancy; Bristol Myers Squibb/Celgene: Consultancy; Bluebird Bio: Consultancy, Research Funding; Allogene: Consultancy, Research Funding; Amgen: Consultancy. Alsina:BMS, Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Research Funding. Sborov:Abbvie: Consultancy; Bioline: Consultancy; Sanofi: Consultancy; BMS: Consultancy; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy. Wagner:Abbvie Inc.: Other: Partner is currently employed as a Medical Science Liaison . Hashmi:JANSSEN: Consultancy; KARYOPHARM: Speakers Bureau; GSK: Speakers Bureau; Sanofi: Consultancy, Speakers Bureau; BMS: Consultancy. Atrash:Celgene: Honoraria, Speakers Bureau; GSK: Honoraria, Research Funding; Takeda: Honoraria; Sanofi: Honoraria, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Research Funding. Simmons:Kite/Gilead: Speakers Bureau. Ferreri:Affimed: Current equity holder in publicly-traded company; Sanofi: Membership on an entity's Board of Directors or advisory committees. McGuirk:In8bio, Inc.: Other: IIT Clinical Trial; BMS: Consultancy, Honoraria, Speakers Bureau; Nextar: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Magenta Therapeutics: Consultancy, Honoraria, Research Funding; Allovir: Consultancy, Honoraria, Research Funding, Speakers Bureau; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; Sana: Honoraria; Orca Bio: Research Funding; CRISPR Therapeutics: Consultancy; Juno Therapeutics: Consultancy, Honoraria, Research Funding. Sidana:Prothena: Honoraria; Sanofi: Consultancy; Oncopeptides: Consultancy; Allogene: Research Funding; Janssen: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Magenta Therapeutics: Consultancy, Research Funding. Hansen:BMS IMW Ide-Cel Academic Advisory Board: Membership on an entity's Board of Directors or advisory committees; OncLive: Honoraria; Survivorship: Honoraria. Patel:Janssen, Celgene/BMS, Caribou Sciences, Arcellx, Cellectis, Merck, Pfizer, Karyopharm, Oncopeptides: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.